

「再生醫療」包括細胞加工製劑療法,免疫細胞療法,幹細胞療法及組織工程學。我們邀請了七位這個領域的專家學者,分享他們的研究成果與看法。
拋磚引玉,期待「再生醫療」能成為正統醫學的主流之一,吸引更多的學院派(Academic) 醫療人員的參與投入,造福人群、嘉惠社會、厚實國家!
李威傑教授
臺北中心綜合醫院減重/代謝手術中心,臺灣, 台北第二型糖尿病的精準代謝手術療法 摘要
Precision Metabolic Surgical Treatment for Type II Diabetes
Obesity and associated type 2 diabetes mellitus (T2DM) is becoming a serious medical issue worldwide. Bariatric surgery has been shown to be the most effective and durable therapy for the treatment of morbid obese patients. Increasing data indicates bariatric surgery, played as metabolic surgery, is an effective and novel therapy for not well controlled obese T2DM patients. However, not all T2DM patient can benefit from metabolic surgery. A precision metabolic surgery was developed for the success of T2DM treatment and to avoid those who might have success by metabolic surgery. The status and future of precision metabolic surgery can be classified into 3 major fields. 1) A safe treatment: Improvement in technique and accumulation of experience has made metabolic surgery a very safe and minimal invasive surgery. The safety profile of laparoscopic metabolic surgery is compatible with laparoscopic cholecystectomy now. 2) New metabolic surgery: Laparoscopic sleeve gastrectomy (LSG) is the leading bariatric surgery but less effective as a metabolic surgery than bypass procedure. Other than RYGB, one anastomosis gastric bypass (OAGB) and Duodeno-jejunal bypass with sleeve gastrectomy (DJB-SG) have been accepted as treatment modalities for obese patient with T2DM. Recent studies demonstrated that gut hormone, microbiota and bile acid change after metabolic surgery play important roles in durable weight loss as well as in T2D remission. Length of BP limb and counting of the small bowel length is realized to be important in designing metabolic surgery. 3) Individualized treatment: Patients who may benefit most from metabolic surgery was those with high risk of T2D complication and high response rate to metabolic surgery, such as young onset T2D Asian. A novel diabetes surgical ABCD score, a simple system for predicting the success of surgical therapy for T2D, may be adopted for patient selection as well as procedure selection.
Key Words: precision metabolic surgery, type II diabetes mellitus, obesity
楊崑德教授
馬偕兒童醫院、陽明交通大學、國防醫學院,臺灣, 台北免疫治療--從體液、細胞到胞外體治療 摘要
Immunotherapies from Humoral, Cell to Exosomal Therapies
Humoral Immunotherapies
Cell Therapies
Cell-free Exosomal Therapy of Mesenchymal Stem Cells (MSCs)
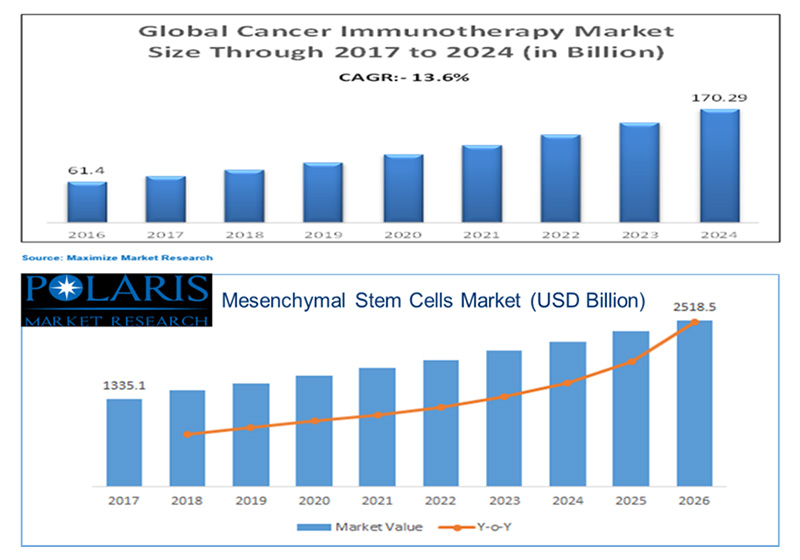
Key Words: immunotherapies, humoral immunotherapies, cell therapies, exosomal therapies
巫康熙教授
中山醫學大學附設醫院 小兒血液腫瘤科,臺灣, 台中從造血幹細胞到間質幹細胞-從基礎到臨床 摘要
From Hematopoietic Stem Cells to Mesenchymal Stem Cells: From Bench to Bedside
Hematopoietic stem cells (HSC) transplantation is now used worldwide in the treatment of many malignant and non-malignant diseases. HSC can be obtained from bone marrow (BM) aspiration, mobilized peripheral blood (PB), or umbilical cord blood. HSC in cord blood have the advantage of tolerance of larger histocompatibility mismatch due to their less immunocompetence. However, the disadvantage of limited cell numbers in cord blood need to overcome. HSC obtained from mobilized PB is favored because it is less stressful than BM aspiration for the patients. Usually, HSC circulates in a very small number in the PB. Therefore, mobilization of HSC from the BM to the PB is an essential part of HSC transplantation. After collection of HSC in PB, HSC will be cryopreserved until transfusion.
In addition to reconstitute the hematopoietic system, the HCT is supposed to affect other tissues such as liver, heart or neurologic tissues. HSC obtained from mobilized PB have found effective in the treatment of cardiac diseases and neurologic diseases. Favorable clinical outcomes strongly indicate the long-term benefit of mobilized HSC transplantation for retrieval from critical limb ischemia. Successfully HSC gene therapy have been reported in hereditary diseases such as thalassemia major. HSC in regenerative medicine will be very promising.
Mesenchymal stem cells (MSC) can be obtained from bone marrow, adipose tissue, teeth, or fetal tissues. However, harvesting MSC from bone marrow involves an invasive and painful procedure. Our team demonstrated that umbilical cord-derived MSC (UCMSC) could proliferate faster than bone marrow-derived MSC (BMMSC), suggesting that umbilical cords may be considered a good source of MSC for clinical use. The pleiotropic properties of MSC that include immunomoduation, antiapoptosis, angiogenesis, growth factor production, neuroprotection, and anti-fibrosis provide a broad spectrum for their potential in disease therapies. MSC have been clinically applied to immune disorder, myocardial injury, osteoarthritis, pulmonary disease, liver disease, diabetes and stroke, and so on.
Our team are very interested in the immunomodulation of MSC. We found that fetal-type MSC had greater immunosuppressive effects than adult-type MSC, and we used UCMSC to treat patients with severe acute graft-versus-host disease effectively and safely. Our team found that the mechanisms of immunomodulation of MSC were associated IL-6, regulatory T cells, Toll-like receptor, and Th2 in animal models. Like HSC, MSC is one of stem cell sources for regenerative medicine.
Key Words: hematopoietic stem cells, mesenchymal stem cells, immunomoduation, regenerative medicine
邱英明教授
國家衛生研究院 細胞及系統醫學研究所,臺灣, 苗栗市協調神經幹細胞之多功能性以運用於神經再生醫療 摘要
Regulating Multipotency of Neural Stem Cells in Neuro-regenerative Therapy
Nerve injury of the central nervous system and peripheral nervous system still poses a major challenge in modern clinics. Understanding the roles of neurotrophic factors and their molecular mechanisms on neuro-regeneration will not only benefit patients with neural damages but could potentially treat neurodegenerative disorders. We previously showed that human FGF1 could improve sciatic nerve injury in rats, likely through FGF1-induced neurogenesis. We now showed that human IL12 p40-p40 homodimer (hIL12p80) within PLA and PLGA conduits improved the sciatic nerve regeneration in mice. As such, the group of PLA conduit with NSCs and hIL12p80 (CNI) showed the best recovery than the other groups in sciatic functional index (SFI), compound muscle action potential (CMAP) and Rotarod performance analyses. In addition, the CNI group had a speedier recovery, outperforming the other groups in SFI and Rotarod performance tests beginning at the fourth week post-surgery. Immunohistochemistry showed that the CNI group increased the diameter of newly regenerated nerve two-fold (P < 0.01). In vitro studies showed that hIL12p80 stimulated differentiation of mouse NSCs to oligodendrocyte lineages through phosphorylation of Stat3. Further, implantation using PLGA conduits (C2.0 and C2.1) showed better recovery in Rotarod test and CMAP than using PLA conduits in FVB mice. In B6 mice, the group with C2.1+NSCs+hIL12p80 (C2.1NI) not only promoted sciatic functional recovery but also reduced the rate of experimental autotomy. These results suggested that hIL12p80, combined with NSCs, enhanced the functional recovery and accelerated the regeneration of damaged nerves in the sciatic nerve injury mice. It is likely that the synergistic effects of FGF-1 induced neurogenesis and IL12-induced myelinogenesis could optimize the effect of nerve repair. Our findings could further shed light on IL12’s effect in rectifying the oligodendrocytes’ defects in neurodegenerative diseases, such as amyotrophic lateral sclerosis and multiple sclerosis.
Key Words: sciatic nerve injury, FGF1, IL12
楊慕華教授
1.國立陽明交通大學 臨床醫學研究所,台灣, 台北大腸直腸癌幹細胞和微環境間之交互作用 摘要
Interplay between Colorectal Cancer Stem Cells and the Tumor Microenvironment
Cancer stem cells (CSCs) have been shown to be responsible for the tumor initiation, metastasis, and therapeutic resistance of colorectal cancer (CRC). In recent year, we focus on the mechanisms for engendering stemness of CRC and the interplay between tumor microenvironments and colorectal cancer stem cells (CRCSC). We found that the EMT transcriptional factor Snail upregulates IL-8 to induce stem-like properties of CRC. Snail coordinates symmetrical division of CRCSC for expanding the stem cell pool of late-stage CRC. Furthermore, we found that the chromatin modifier ARID3B activates the expression of intestinal stem cells genes and PD-L1 through a noncanonical Notch pathway. Regarding the communications between CRCSC and microenvironments, we demonstrate that the CRCSC-derived exosomal miR-146a is a major exosomal miRNA for enriching tumor-infiltrated neutrophils. CRCSC-secreted exosomal tri-phosphate RNAs induced the expression of IL-1β to sustain neutrophil survival. CRCSC-secreted CXCL1 and CXCL2 attracted CRCSC-primed neutrophils to promote tumorigenesis of CRC cells. In summary, our studies demonstrate the interplay between CRCSC and tumor-infiltrated neutrophil through tumor-derived exosomes, which sustains the immunosuppressive microenvironment of CRC for tumor progression.
Key Words: cancer stem cell, epithelial-mesenchymal transition, tumor microenvironment
許益超教授
馬偕醫學院 聽力暨語言治療學系,臺灣, 新北市幹細胞醫學於感音神經性聽損之治療應用 摘要
Stem Cells and Stem Cell-Derived Molecules for the Treatment of Hearing Loss
Millions of people worldwide suffer from hearing loss, predominantly sensorineural hearing loss (SHL) due to the damage or loss of the sensory hair cells (HCs). Inner ear HCs are responsible for converting sound vibrations into electrical signals, transmitted through spiral ganglion neurons (SGNs) to the brain. Mammalian HCs and SGNs cannot self-regenerate. Once these HCs or SGNs suffer from stress or damage, the number of HCs and SGNs in the cochlea starts to decline, resulting in SHL. Currently, only SHL preventive approaches exist and no approved chemical or biological drugs. Cisplatin is a chemotherapeutic drug used to treat many solid tumors, such as lung cancer, head and neck cancer, ovarian cancer, bladder cancer, testicular and uterine cancers. However, its use tends to result in nephrotoxicity and ototoxicity. Ototoxicity of cisplatin occurs due to depletion of antioxidant substances and increased lipid peroxidation, with resulting apoptotic damage of the HCs and SGNs. Previous efforts utilized insulin growth factor, brain-derived growth factors, glial derived growth factor or other neurotrophins to treat SHL through promoting the survival of HCs or neurite extension of SGN. For the utilization of stem cells to treat SHL, the otic progenitor cells (OPCs), neural progenitor cells (NPCs) and mesenchymal stem cells (MSCs) are considered to be beneficial. OPCs are a good cellular sources for the regeneration of HCs. NPCs or NPC-derived molecules are beneficial for the regeneration of SGNs to facilitate the efficacy of cochlear implant. MSCs and MSCs-derived molecules can promote tissue repair and tissue remodeling through secreting many anti-inflammatory molecules, miRNAs and growth factors. In my talk, the possible contribution of different stem cells and stem cell-derived molecules for the improvement of hearing loss will be presented and discussed.
Key Words: sensorineural hearing loss, stem cells
李啟賓研究員
精準細胞免疫治療中心 中國醫藥大學附設醫院,臺灣, 台中利用嵌合抗原受體T細胞針對各類腫瘤細胞表面之癌胎硫酸軟骨素抗原之免疫療法 摘要
CAR-T Cell-Based Therapy Directed against the Universal Tumor Antigen Oncofetal Chondroitin Sulfate
Chimeric antigen receptors (CARs) have shown remarkable success against CD19-expressing hematological malignancies. However, their role in solid tumors with dismal prognosis such as metastatic pancreatic cancer, ovarian cancer, and lung cancer has not yet been confirmed. Effective immunotherapy for solid cancer requires (1) identification of novel CAR targets expressed at high levels on tumor tissue, but not on normal tissues, and (2) successful ablation of the immunosuppressive tumor microenvironment (TME), allowing CAR-T cells to infiltrate and engage with the tumor. Here, we introduce the glycosaminoglycan oncofetal chondroitin sulfate (ofCS) as a novel universal cancer target for CAR-T cell therapy. VAR2 is a member of the P. falciparum erythrocyte membrane protein 1 family, and is expressed by erythrocytes when infected with plasmodium falciparum. It was identified as binding with high affinity to a specific chondroitin sulfate glycosaminoglycan (GAG) chain consisting of long, linear carbohydrates made up of repeated disaccharide units expressed on the surface of syncytiotrophoblasts in the placenta, facilitating infection during placental malaria. we can overcome the lack of suitable cancer-specific CAR-T targets utilizing the malaria protein VAR2 which binds to the universal cancer antigen oncofetal chondroitin sulfate (CS). Our group designed ofCS-targeting CAR-T cells with the ofCS-specific binding moiety of the protein VAR2. We found ofCS expressed in a large variety of cancer cells irrespective of their molecular state and origin, but not detectable in normal cells.We found ofCS expressed in many cancer cells irrespective of their molecular state and origin, but not detectable in normal cells. We observed that VAR2-CAR-T cells lysed 90% of CHX-1990-GFP cells within 24-h in cytotoxicity assays. The VAR2-CAR T cells effectively clear pancreatic cancer cells orthotopically implanted in the pancreas of C57BL/6J mice, and tumor volume is reduced by 85% after treatment on 18 days and prolonged survival of treated mice. Thus, our results demonstrated the anti-tumor efficacy of VAR2 CAR T cell therapy against pancreatic cancer, suggesting its therapeutic potential.
Key Words: CAR-T cell therapy, universal cancer, tumor microenvironment; oncofetal chondroitin sulfate glycosaminoglycan.
嵌合型抗原受體(Chimeric antigen receptors, CARs)目前在對抗表達 CD19 的血液惡性腫瘤方面取得了顯著的成功。但CAR治療在固體癌症上卻無顯著的效果,尤其在轉移性胰腺癌、卵巢癌和肺癌預後不佳等實體瘤中的作用尚未得到證實。因此,針對實體癌有效免疫細胞治療需要包含下列幾點面向 (1) 具有識別在腫瘤組織上高表達的新型嵌合型靶標且不表達在正常組織上 (2)成功消融免疫抑制性腫瘤微環境 (TME),使 CAR-T 細胞能浸潤並與腫瘤接觸。有鑒於此,本團隊找出一種普遍會表現在癌細胞表面的醣類分子,稱之為腫瘤胚胎硫酸軟骨素(ofCS)來做為CAR-T細胞免疫治療之新標點。VAR2蛋白是惡性瘧疾原蟲紅細胞膜蛋白 1 家族的成員,在感染惡性瘧疾原蟲時會經由紅細胞表達,已被鑑定出與特定的硫酸軟骨素糖胺聚醣鏈具有高親和力結合,該鏈由長的線性碳水化合物組成,其碳水化合物由胎盤融合滋養層細胞表面上表達的重複二糖單元所組成,有助於胎盤瘧疾期間的感染能力。癌胎兒硫酸軟骨素抗原表達於多數癌症細胞表面,利用此特性便可與瘧疾蛋白VAR2之鍵結作為CAR-T細胞上的標靶,進而克服缺乏合適的癌症特異性CAR-T細胞標靶的問題。本團隊利用來自小鼠活化的T細胞設計出直接或間接表達VAR2的CAR-T細胞,具有辨識癌細胞上的ofCS能力進而將其毒殺摧毀。我們發現多種癌細胞表面ofCS過度表達,但無論其分子狀態和來源為何,正常細胞表面卻無法被檢測到,這說明此種特異性可避免CAR-T細胞誤殺正常細胞,因此降低了CAR-T細胞治療上安全的隱憂。我們也觀察到在VAR2-CAR-T細胞在in-vitro的胰臟癌細胞毒殺測試中,24小時內就能毒殺90%以上的胰臟癌細胞,並且在小鼠胰臟原位癌模式中經過18天後VAR2-CAR-T細胞能降低約85%的固態腫瘤體積並延長其生命。因此,我們的結果證明了 VAR2-CAR-T細胞免疫療法對胰腺癌的抗腫瘤功效與其治療潛力。
關鍵字: 嵌合抗原受體T細胞治療; 各類癌症;腫瘤微環境; 腫瘤胚胎硫酸軟骨素糖胺聚醣
定大國際法律事務所
定大會計師事務所