

Senior Medical Director, Medical Affairs, Oncology & Hematology at Worldwide Clinical Trials. Business Address: Buenos Aires, Argentina
1. Observational Study
Daniel Gandia M.D., reviewing Ben-Arie E. et al. Medicine 2022; 101 :38 (e30716).
Oral Cancers, within Squamous Cell Head and Neck Cancers, are a problem of major concern in many parts of the world.
Traditional Chinese Medicine (TCM), such as physical ones (Acupuncture) or herbal ones are well-known millenary and are widespread in the treatment of several diseases such as Cancer.
The authors performed an elegant retrospective longitudinal cohort study with data obtained from the Taiwan National Health Insurance Research Database (NHIRD) and from the Longitudinal Health Insurance Database 2000.
The primary patient population, compromise those diagnosed with oral cancer between 2000 and 2009.
In both patient populations, the Non-TCM (N:821) and in the TCM one (N:130) there was a prevalence of the male patient population. Median ages were similar also, and in both the most common comorbidities were hypertension and Diabetes.
The top 10 TCM single herbs and herbal formulas used were: Xuán sheng (Radix Scrophulariae), Yàocǎo shíhú (Herba Dendrobii), Mài dōng (Ophiopogon Japonicus), Qiánmá de guǒshí (Fructus Crataegi), Rhizoma Ligustici Wallicii, Rhizoma Pinelliae Preparatum, Rhizoma Atractylodis Macrocephalae, Cortex Albizziae, Cortex Lycii and Bulbus Lilli.
The study has some caveats, such as the study sample size, which was imbalanced with respect to both patient populations, and the number of patients were not sufficient.
The TCM group demonstrated a significantly higher 5-year survival rates when compared with the non TCM users (HR: 0,57, 95%, CI:o.40, 0.82; log-rank test P: .001). These findings also show that the TMC users had a significantly higher 12-year survival measurement when compared to the non TCM users (HR: 0,64 95% CI: 0.48, 0.85; log-rank test P: .01).
Comment: This study gives us, clinically applicable information on the use of TCM in oral cancer patients in Taiwan.
This observational study, also provides important data on the estimations of the 5- and 12-year survival for oral cancer patients in Taiwan.
TCM in oncology is integrative and complementary of the traditional Cancer treatments such as Chemotherapy, radiation therapy, Targeted therapies and Immunotherapy. It is of great interest to develop studies with TCM in the western countries, in randomized fashion design in order to prove the worth of TCM in these other parts of the world also.
2. Original Article
• Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death.
Daniel Gandia M.D., reviewing Bretthauer M. et al., N Engl J Med 2022, October 9.(https://doi.org/10.1056/NEJMoa2208375)
No large randomized, controlled studies have been conducted to examine mortality outcomes from colonoscopy screening for colorectal Cancer (CRC) until now. In this study, 85000 previously unscreened people (age range 55-64) in Norway, Poland, and Sweden were randomized in a 1:2 ratio to receive an invitation to undergo colonoscopy screening, or to receive no invitation.
Only 42% of the people in the invited group accepted their invitations and underwent colonoscopy; those with polyps had subsequent surveillance at standards intervals. The primary intent-to-screen analysis (which includes all invited people, whether or not they followed through with colonoscopy) revealed the following outcomes at 10 years:-cumulative incidence of CRC was significantly lower in the invited group (0.98% versus 1.20%), likely due to removal of precancerous polyps.
-CRC-related mortality at 10 years was not significantly different in the two groups (0.28% versus 0.31%).
-All-cause mortality at 10 years was identical in the two groups (11.0%)
-Among nearly 12000 screening colonoscopies, no perforations and 15 cases of major bleeding were reported.
In a secondary adjusted per-study analysis a comparison of people who actually underwent screening versus controls, a difference in CRC-related mortality reached significance (0.15% versus 0.30%). This “best Case” outcome would translate to roughly 1 fewer CRC death per 700 screened people.
Take-Home-Message: these results are not straightforward; hence, they probably won’t change the general U.S. preference for colonoscopy to screen for CRC. The editorialists consider the primary findings a small relative reduction in CRC risk and no reduction in CRC-related mortality as “surprising and disappointing”. Results of the per-protocol analysis, which includes only the minority of invited people who actually underwent colonoscopy but introduces bias that randomization was designed to eliminate, are somewhat more compelling.
Moreover, longer follow-up might yield a larger survival benefit. Additional randomized trials to compare colonoscopy and no screening probably won´t be done, but large trials comparing colonoscopy and fecal immunochemical testing are ongoing.
3. Stem Cell and Gene Therapy
Daniel Gandia M.D., reviewing Baloh R.H. et al. Nature Medicine Vol 28, September 2022, pp 1813-1822
Amyotrophic lateral sclerosis (ALS) is a desvasting neurodegenerative disease with no currently curative intent.
Two drugs are approved at the present time, Riluzole and Edaravone, but they are mild disease-modifying agents, with marginal clinical benefits.
Therefore, the avenue of new therapy-types is an unmet medical need. Gene therapy is an emerging and promising one in the future treatment of ALS.
ALS is characterized by the selective loss of upper and lower motor neurons, but dysfunctional astrocytes play a crucial role in the development of the disease. This suggest that healthy astrocytes, exert a protective effect on motor neurons.
Here, Baloh et al, report the first -human trial as a phase 1/2a of this strategy in 18 patients with ALS.
It must to be clarified here, that these patients were at late disease stages and pretreated before.
Baloh et al, created a good manufacturing, ex vivo, allogeneic gene therapy product, named CNS10-NPC-GDNF. They found that transplanted this compound could engraft in the spinal cord, where the cells survived and differentiated into astrocytes. Their work is very elegant and describes, preclinical animal data-including dose-ranging studies, tumorigenicity and toxicology studies, and large animal safety trials- culminating in the phase 1/2a dose-escalation trial in 18 patients with ALS.The cells were transplanted only on one side of the spinal cord, with the non- transplanted leg being used as control. Moreover, it is important to note that this treatment requires only a one-time delivery-as the cells were expected to survive and secrete GDNF for the life of the patient.
At one year of follow-up, the study has not safety signals. One patient experienced a dramatic preservation of function on the treated side at 3 years after transplantation.
Post-mortem tissues were collected from the 13 patients, and, notably, the results showed that grafted cells have survived and expressed GDNF in all 13 of them.
It will be important to clarify whether the therapeutic effect of CNS10-NPC-GDNF is limited to the injection sites, or whether systemic effects are seen.
Comment:it will be a must, to include in this type of studies patients at earlier disease stages and less pretreated ones, in the intent of achieve the best results.
CNS10-NPC-GDNF can be safely administered to the diseased spinal cord and exogenous gene products can be produced for over 3 years without affecting leg function.
This study provides the foundation for future clinical trials in same class.
4. Review Article. Stem Cell & Biochemical Therapy.
•Modelling urea cycle disorders using iPSCs.
Daniel Gandia M.D. reviewing Claire Duff and Julien Baruteau. Npj Regenerative Medicine (2022) 7:56; https://doi.org/10.1038/s41536-022-00252-5
Patients that present with recurrent acute hyperammonemia have a grim prognosis. This metabolic condition can be in relation to inherited congenital urea cycle disease, which comport critical enzymatic deficiencies or related to hepatic insufficiency due to fulminant hepatitis or complicated cirrhosis.
Urea cycle (UC) is performed exclusively in the liver, more exactly in the periportal hepatocytes. Ammonia is produced by the deamination of amino acids and is a highly neurotoxic molecule.
For mention some of the before mentioned deficiencies, these are: CPS1 deficiency, ornithine transcarbamylase deficiency, citrullinemia type I , arginosuccinate lyase defiency, arginase-1 deficiency, citrin deficiency, that are the complete relevant deficiencies ones.
So, in relation to the above-mentioned, two possibilities are on the way: liver transplantation, or the design of new medicine regenerative therapies in order to correct these inborn alterations.
Induced pluripotent stem cells (iPSC) and genome editing technologies have provided an invaluable opportunity to patient-specific phenotypes in vitro by creating patients’ avatar models. Gene therapy was developed for targeting ornithine transcarbamylase deficiency in the nineties, but immunogenicity of the viral adenoviral vector led to tragic consequences.
On the other hand, iPSCs models present intrinsic limitations, particularly for therapeutic application and translation to the clinics. One of the significant challenges faced by iPSCs research is, that despite ongoing improvements in differentiation protocols, the immaturity of iPSCs-derived cells, which remain closer to fetal rather than their fully counterparts. This has been demonstrated in modelling urea cycle disorders, where both wild-type and mutated immature differentiated hepatocytes failed to achieve equivalent functionality with that of in vivo hepatocytes.
But iPSC differentiation provides a unique opportunity to study a genetic disease in a specific genetic and epigenetic background in vitro and screen for therapeutic candidates. This regenerative-medicine cellular type, have improved our knowledge of the pathophysiological mechanisms of several diseases, alongside accelerating progress in our understanding of human physiology at the cellular level.
Comment:Urea cycle disorders -derived iPSCs are progressively expanding their applications in several Medicine areas, from supporting a genetic diagnosis in the genomic era, studying pathophysiology to drug development and validation. iPSCs offers the unique opportunity to implement personalized medicine and perform drug screens.
We can say we are in an embryo nary period, facing with challenges, but also it must be said that is really exiting that Regenerative Medicine is not only to regenerate organs, create organoids and others, but also a new biochemical area of it, challenging and improving “damaged metabolic cellular pathways” such as is urea cycle.
1. Stem Cells (Research-Report)
• JMJD3 activated hyaluronan synthesis drives muscle regeneration in an inflammatory environment.
Daniel Gandia M.D., reviewing Nakka K. et al. Science, Vol 377 (issue 6606), 5 august 2022, pp 666-669
Skeletal muscle, the largest tissue in the human body, has regenerative potential after injury. This is due to the capacity of muscle stem cells (MuSCs), that are normally in a dormant or quiescent state (G0 cell cycle phase) and are activated by signals such as dying cells, inflammatory cells, leading to the muscle regenerative activity.
Even when, it is not known how MuSCs adapt to the modified niche, the authors here, made an outstanding discovery.
MuSCs activate the production of hyaluronic acid, a glycosaminoglycan ubiquitous in the extracellular matrix, which is required to overcome the inhibitory inflammation signaling from the damaged niche, exit quiescence and, initiate the muscle tissue repair.
The cell destinies transitions induced by muscle injury, are associated with epigenetic changes of the MuSCs.
After tissue injury, MuSCs up-regulate the expression of JMJD3, a protein which derepresses the hyaluronan synthase 2 (Has2) promoters, the resulting Has2 protein increases hyaluronic acid production.
This extracellular matrix molecule protects MuSCs from inhibition by inflammatory cytokines secreted by macrophages, allowing MuSC proliferation and organ repair.
Hyaluronic acid is needed only when MuSCs are exposed to an inflammatory microenvironment, and hyaluronic acid and its receptors could be signals in the injury niche that promote regeneration. Latter during the muscle regeneration process, inflammatory cells modify their gene expression pattern and their metabolic programmes. They stimulate MuSC fusion and differentiation to build new skeletal muscle tissue. Both processes are required for the resolution of inflammation, tissue restoration and the return to homeostasis.
As a relevant aspect to study in the future, we must address if whether hyaluronic acid degradation contributes to MuSCs return to quiescence upon muscle damage repair.
Comment: several skeletal muscle diseases are known, many of them with a genetic and hereditary basis. Some of them and others in -type, can be potentially investigated with the “muscle repair approach”.
Hyaluronic acid, is a central molecule in all these repair processes, and still is not known and warrants further investigations, if whether an altered hyaluronic acid production by MuSCs contributes to the abnormal deposition of extracellular matrix constituents and the reduced muscle tissue regenerative potential in aging.
2. Tissue Engineering (Review)
*Building gut from scratch-progress and update of intestinal tissue engineering
Daniel Gandia M.D., reviewing Tullie L. et al. Nature Reviews / Gastroenterology & Hepatology, Vol 19, July 2022, pp 417-431
Tissue engineering is a complex technology that requires a collaborative effort across disciplines such as: biology, internal medicine, biotechnology and engineers. Regenerative medicine, tries at its best to restore cell, tissue or organ function in diseases which clinically are at end-stage phase.
The advances in the field of intestinal tissue engineering (ITE) are huge since the first milestone in 1981 with the discovery of intestinal pluripotent stem cells, highlighted in 2004 with the discovery in mice of the organoid unit for intestinal failures, and with the 2021 milestone of the development of small intestine organoids delivered onto colonic scaffold in vivo.
An unmet medical need in gastroenterology is a curative treatment for short bowel syndrome, a condition that comports insufficient nutrients absorption at the intestinal epithelium. Even when the disease is not very frequent, the only treatment for these patients is intravenous parenteral nutrition, a supportive care and continuous measure for these patients to live.
Tullie et al, updates us in this article with the exciting advances made in the field of ITE over the last 10 years.
Tissue-engineered small intestine grafts could be generated from different cellular components such as organoids, mesenchyme and neural crest cells derived from embryonic stem cells, induced pluripotent stem cells and primary cells.
The sources for the scaffold include decellularized intestinal tissue or synthetic or natural polymers.
The strategies of ITE nowadays comports cell-based therapies, cell combinations seeded onto scaffold to produce mucosal grafts and mucosal repurposing to generate a small intestinal zed colon.
Safety grafts is the most important issue, that can transition in a correct way, the bench to bedside of transitioning tissue engineering.
The ultimate aim of the generation of a full-thickness functional intestinal graft for transplantation is an opportunity for the treatment of short bowel syndrome. But, even when this step is a considerable way off being achieved, the progress towards generating individual intestinal components offers the opportunity to employ targeted engineering strategies. It is likely so, that the first clinical translatable therapies will be cell-based or partial reconstructions, rather than full-thickness tissue engineering small intestine, and perhaps in some cases will prevent causative pathologies progressing to irreversible intestinal failure.
Take-home-Message: Intestinal tissue engineering is a fascinating field of regenerative medicine in a continuum of progress.
The interaction of a multidisciplinary team is a maximal need in order to work -out the major concern problem of short bowel syndrome.
This review article highlights some topics in ITE, and concludes that at the present time, the best approach for this, is the continuous development of the personalized tissue engineering small intestine grafts and cell selection derived from intestinal cells, from pluripotent stem cells differentiation in vivo or in vitro or from a mixture of the two.
Tissue intestinal engineering is an essential not only for small bowel diseases but for colorectal ones, mainly in “necessary radical total colectomies” in colorectal cancers.
3. Organoids (Research Article)
Tissue geometry drives deterministic organoid patterning
Daniel Gandia M.D., reviewing Gjorevski N. et al. Science, vol 375, eaaw9021 (2022). DOI: 10.1126/science. aaw9021
Stem-cell-derived organoids are in vitro tissue and organ mimetics that comports a great potential as models for human diseases and organ development, and as platforms for novel drugs discovery and diagnostics, and lastly for the design of cell and gene therapies.
The resulting structures of organoids, would allow both understanding the underlying morphogenetic mechanisms and building models that bear higher likeness to the natural counterparts.
The best described organoid-system nowadays are the location and number of crypt-like domains in intestinal mouse organoids.
Gjorevski et al, describes here their development in the matter.
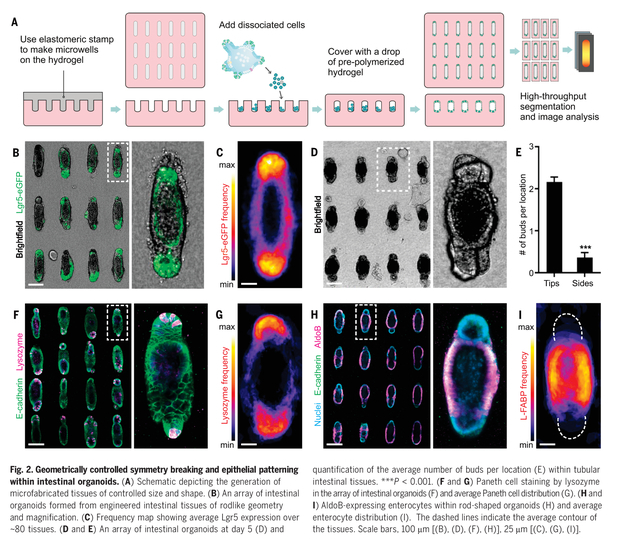
Taking into account the development in vivo, they complemented organoid self-organization with external regulation. They attempted to control the patterning and morphogenesis of intestinal organoids via the physical properties and, in particular, the initial geometry of the tissue itself.
Bioengineering strategies were developed to extrinsically control the self-organization process of intestinal stem cells through in situ photopatterning of hydrogel mechanics and hydrogel microfabrication (see Figure 2). Epithelial intestine cells or enterocytes are several and different, and the identification of the underlying mechanism of epithelial patterning, was studied by using the predictability of organoid development.
Their data suggest that in vivo-like tissue geometries can manage stereotypical epithelial patterning by establishing reproducible local differences in cell packing and morphology. Spatial heterogeneous mechanosensing/transduction (YAP) and Notch signaling were associated for the differences in cell shape. They in turn specify “crypt” and “villus”-like domains by localizing Paneth cell differentiation and suppressing stem cell destinies, respectively. Spatial cell variations in morphology dictated by tissue geometry thus render a normally random process highly stereotypical. The authors exploited these insights to build macroscopic organoids that resembled the periodic crypt-villus architecture of the native intestinal epithelium.
Need-to-know: Intestinal histology is complex, comporting different cells, but mainly the crypt and villus morphology are a must to be considered when structures-like of them are going to develop by organoids.
These organoid cultures can be used to address and answer questions not ready addressable by the existing organoid and animal models, and may enable the bench-to-beside of organoid technology.
4. Immune Cells (Research Report)
*CAR T cells produced in vivo to treat cardiac injury
Daniel Gandia M.D., reviewing Rurik J.G. et al. Science Vol: 375, pp 91-96 (2022) 7 January 2022
Fibrosis is a pathogenic process present in several organs and which comports fibrotic proteins in the extracellular organ’s matrix.
Depending on the organ-tissue, it sometimes conduces to failure and in the heart the process is the previous one to congestive heart failure that in cases leads to organ transplantation.
Therapies targeting fibrosis is still a clinical unmet need.
A promising therapy for it, is here described by Rurik et al.
He describes a new way approach to eliminate activated fibroblasts by harnessing the power of engineered T cells. Lipid nanoparticles (LPNs) carrying a messenger RNA (mRNA) that encodes a chimeric antigen receptor (CAR) are used to generate CAR T cells in mice, yielding a therapeutic T cell population that is capable of ablating pathogenically activated fibroblasts and attenuating cardiac fibrosis. CARs are synthetic receptors that allow immune cells- usually T cell lymphocytes to recognize targeted antigens and initiate antigen-specific immune responses.
CAR T cell therapy has shown awesome clinical results in hematologic malignancies, with long-term survivors and even cures, but the long-lasting beneficial effect present with this therapy in cancer patients has not to be present in anti-fibrosis heart therapy. The effects of therapy have to be transient in the heart injury process for safety concerns. This is achieved, for as mentioned before, mARN is used in the technique of building CAR and its effect is transient.
The decline of CAR T cells in mice achieve undetectable levels within 7 days. Using in mice the hypertensive cardiac injury model, the authors demonstrate that transient Fibroblastic CAR T cells substantially reduce ventricular fibrosis and improve various cardiac functions, as assessed by electrocardiography.
The above-mentioned findings validate the use of this therapy modality in heart fibrotic disease and outside of the classic indications of it in Oncology. For a clearer dissected understanding see figure below.
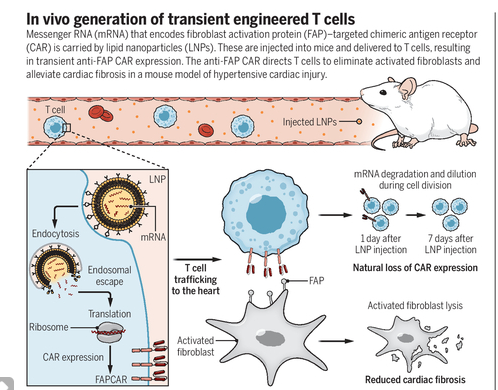
Comment: This exiting manuscript, reveals that Immunotherapy CAR T cells- type, comports a possibility of treatment for other diseases and not only blood cancers.
Heart failure is the end-organ-stage part of this disease with unmet clinical needs.
This well-designed and well conducted therapy preclinically in mice will without doubt be an essential in the future treatment of this otherwise chronic and finally deadly disease as is heart failure due to the underlying process of fibrosis.
1. Original Article
*Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma.
Daniel Gandia M.D., reviewing Gallego Pérez-Larraya J. et al. N Engl J Med 2022; 386:2471-81
Pediatric patients with diffuse intrinsic pontine Glioma (DIPG) have a median overall survival (OS) of <1 year and comports limited effective treatment options. The authors here, present data from a Phase 1 Trial that demonstrate that the intratumorally administered oncolytic virotherapy with DNX-2401 virus (adenovirus-type) is safe, with evidence for long-term activity in a subset of patients.
A total of 12 children, with a median age of 9, with newly diagnosed DIPG, received a single dose of 1 x 1010 particles of DNX-2401 (4 patients), increasing to 5x 1010 following preliminary confirmation of safety at the lower dose (8 patients), administered intratumorally immediately after biopsy sampling.
The majority (11 of 12) received subsequent Radiation therapy.
The primary end-point was to determine the safety profile of the oncolytic viral therapy.
Three clinical serious adverse events were observed: transitory grade 3 hemiparesis, probably due to biopsy sampling, and grade 3 neurological deterioration of bilateral oculomotor paresis and tetra paresis in the same patient. Common, mostly lower-grade adverse events included headache, neurological deterioration and vomiting, each in 75 % of the patients.
Three patients presented with partial responses (defined according to RANO criteria) with stable disease observed in 8 patients (Disease control rate of 91%). The median OS duration was 17.8 months, with 3 patients remaining alive at 19.6, 31.4 and 33.5 months at the latest follow-up cutoff.
Owing to difficulties with repeat biopsy sampling, longitudinal data on immune-cell modulation are limited to 1 patient only.
Nonetheless, this analysis demonstrated several changes suggesting activation of antitumor immunity (such as increased numbers of tumor-infiltrating macrophages and pro-inflammatory chemokine expression).
Comment: These excellent findings provide evidence for tolerability and preliminary efficacy of this type of oncolytic viral therapy with DNX-2401 in DIPG patients.
This entity is a difficult-to-treat-disease with still unmet medical therapy needs.
Clinical data from larger cohorts of patients are eagerly awaited.
This type of therapy is also a window of opportunity for the treatment of relapsed adult glioma patients.
2. Original Article
*Molecular Features of Cancers Exhibiting Exceptional Responses to Treatment
Daniel Gandia M.D., reviewing Wheeler D.A. et al. Cancer Cell 39, 38-53, January 11, 2021
Even when many advanced tumors are currently cured with different medical therapies maneuvers, namely Chemotherapy, Immunotherapy, Targeted Small Molecule treatments, many of them relapse due to different mechanisms of tumor resistance.
One of the main foci nowadays in basic and clinical research is to explore how to work – out those mechanisms of tumor resistance, which have complex underlying molecular features.
On the other hand, it is noteworthy that some peculiar subset of patients, present with excellent clinical tumoral responses, namely complete or major partial ones and with extended periods of overall survivals.
These patients are called “exceptional responders”. Even when it is not a unique definition for them, this one has to comport disease-specific, stage-specific and treatment -course specific traits.
Wheeler et al analyze in this manuscript, the molecular profiling of this subset of patients. This profiling was in a multiplatform genomics of 110 patients with exceptional response to therapy profiled. They identify some putative molecular mechanisms and biomarkers that explain this survival phenotype in approximately 23 % of cases within the 110 subsets.
Tumors with high epidemiologic impact were dissected from this genomic point of view, as to mention some of them: brain malignant cancers, breast cancer, colorectal cancer and other gastrointestinal tumors, biliary tract cancers, lung cancer, ovarian and pancreatic cancer. Most of these exceptional responders presented with complete responses and with prolonged periods of overall survivals ranging from 10 to 145 months.
The proposed putative mechanisms studied and found were: DNA damage processes (e.g., synthetic lethality), signaling pathways alterations (e.g., oncogene addiction to drugs), tumoral immune microenvironment and responses, prognostic specific biomarkers (e.g., ATR expression in brain cancers).
Comment: with the enormous Cancer Science in development and already developed, mainly related to genomics and proteomics, these kinds of patients are to be strictly seeked, for the prognosis and clinical outcome is very different in relation to patients with same kind of tumors but with relapses or resistant to different therapies modalities.
Without doubt all the above-mentioned is an eye-opener in Cancer therapy and genomics and these patients are to be deeply studied and published in the literature in order to help other physicians who have similar kind of exceptional responders.
Daniel Gandia M.D., reviewing Poch Ch M. et al. Nature Cell Biology, Vol 24, May 2022, pp 659-671
Within Cardiovascular diseases, acute myocardial infarction and heart failure, are two entities of major clinical and public health concern.
Even when many preclinical and clinical research in cell therapy has been performed up to date, the results are inconsistent or at best moderately positive effects are reported. These studies mainly used bone marrow cells and the lack of efficacy may be merely due to the inability of these cells to restore cardiac function, or as there is no cabal understanding of the mechanism of action. Cell therapy on heart repair is an area in expansion, knowing nowadays many of the molecular involved mechanisms. For cell therapy to be effective, a reproducible source of (engineered) cells capable of self-assembling into myocardium or a myocardium-like structure without causing arrhythmias or having tumorigenic potential are required.
Human ventricular progenitors (HVPs) may resolve these requirements. HVPs are cardiac progenitor cells derived from human embryonic stem (ES) cells that have initiated the differentiation process towards cardiomyocytes. To gain mechanistical insight into the mode of action of HVPs, the authors used ex vivo heart slices of left ventricular tissue of adult non-human primates, a species evolutionary close to humans to improve predictive value for potential translation to patients.
Radiofrequency ablation was utilized to induce a local injury site in this model. Seeding either radiolabeled HVPs or cardiomyocytes (CM) on the opposite site of the tissue slice indicated a migratory capacity of the HVPs which was absent in the CMs. The migration of HVPs initiated remuscularization in the injured area, which corresponded to a reduction in scar size and improved contractile function as compared to the CM-treated slice tissues. So, HVPs contain an enhanced capacity to migrate into the injury area to restore the damage. In the proposed mechanism of action of HVP-mediated cardiac cell therapy, CXCL12 is secreted after injection by activated cardiac fibroblasts and induces CXCR4-expressing HVPs to migrate towards the injury site. Then, HVPs induce the repulsion of cardiac fibroblasts in a peculiar and complex manner, followed by remuscularization of part of the injured area. All the above-mentioned last, was analyzed by single-cell transcriptomics analysis.
Comment: this study complements a series of manuscripts showing that embryonic or progenitor cardiomyocytes can muscularized parts of the infarcted heart and improve cardiac function in large animal models. The success of cardiac cell therapy using HVPs must next, jump from the preclinical-bench side to the bedside of patients in order to treat formerly, cardiac threatening diseases such as myocardial infarct or congestive heart failure, two “epidemic diseases” with still some unmet medical needs.
* FoxO3 restricts liver regeneration by suppressing the proliferation of hepatocytes
Daniel Gandia M.D. reviewing Chi-Qian Liang et al. npj Regen Med 7, 33: 1-16, (2022)
Liver regeneration has been focused on the past and present as a cutting-edge area within Hepatology. Many diseases produce the need of liver regeneration such as cirrhosis, hepatectomy, metastases hepatic surgery, chronic hepatitis, and so on. In the past, there was an interesting theory that mentioned a peculiar kind of proteins termed “chalones” which were involved in the regeneration process. Chalones were continuously inhibiting the growth of liver, so they represented a negative proliferative signal. When a partial hepatectomy was performed, the concentration of chalones went down, so now, the liver presented the possibility of regeneration, the chalones inhibiting signal was less. So, upon injury, the liver is capable of substantial regeneration from the original tissue until an appropriate functional size. The underlying mechanisms controlling the liver regeneration processes are still not well elucidated. Previous studies have proposed that the transcription factor FoxO3 is involved in various liver diseases, but its exact role in the regulation of liver regeneration remains largely unclear. Elegant complex studies were performed in mice subjected to 70% partial hepatectomy. The data demonstrate that FoxO3 deletion accelerates liver regeneration primarily by limiting polyploidization and promoting the proliferation of hepatocytes during liver regeneration. RNA-seq analysis indicates that FoxO3 deficiency greatly alters the expression of gene sets associated with cell proliferation and apoptosis during liver regeneration. Fox3 is a transcription factor (well is known about their role in the differentiation of stem cells). FoxO3 promotes the expression of Nox4 that significantly suppressed hepatocyte proliferation and liver regeneration. We demonstrate that FoxO3 negatively controls hepatocyte proliferation through Nox4 upregulation, thereby ensuring appropriate functional regeneration of the liver. We can make the hypothesis that this Transcription factor FoxO3 is the old named “chalones”.
Comment: the mechanisms of liver repair and regeneration, secondarily due to different diseases or procedures is still a nearly missing point in hepatology science. The emerging role of the transcription factors in different diseases mechanisms (Cancer for e.g.) gives a window of opportunity to know more about hepatocyte proliferation and regeneration mechanisms. FoxO3, in this work in knockout adult mice, shows that it negatively impacts in the proliferation of hepatic cells and regeneration processes. FoxO3 negatively regulates hepatocyte proliferation and liver regeneration by promoting Nox4 expression and suppressing Nr4a1 expression, thereby controlling functional regeneration of the liver.
• PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer
Daniel Gandia M.D., reviewing Cercek A. et al. N Engl J Med 2022; 386:2363-76
Rectal Cancer, a very frequent Cancer is primarily managed by Chemotherapy plus Radiation Therapy followed by surgical resection.
Concomitantly with the enormous advance of cellular and molecular Cancer mechanisms, a peculiar subset of rectal cancer patients warrants different novel treatments. This group is the one with the mismatch-repair-deficient mechanism which produces the cancer.
In the setting of metastatic disease these patients are plausible of treatment with Checkpoint immune inhibitors (PD-1 blockade), finding here impressive tumoral responses with these novel agents.
The goal of this Trial was to evaluate the PD-1 blockade in the neoadjuvant clinical setting, that means, patients naïve of other treatments with the intention of evaluation of tumoral responses and local disease control.
A total of 16 patients with mismatch repair deficiency (dMMR), stage II-III rectal cancer patients received Dostarlimab (a checkpoint inhibitor: PD-1 blocker) as monotherapy for 6 months, followed by Chemotherapy and radiotherapy and posterior surgery.
Patients with a complete response (CR)to neoadjuvant therapy received non-operative follow-up only. CR at 12 months or pathological CR (in patients undergoing surgery) were the primary endpoints of this clinical Trial.
At a median follow-up duration of 12 months, all patients included in the efficacy analysis had a clinical CR (objective response rate 100%) This was evaluated by clinical exam (digital rectal examination plus endoscopy), magnetic resonance imaging, F-fluorodeoxyglucose-positron-emission tomography (PET) or biopsy. All evaluable patients remained disease-free at >12 months following the completion of Dostarlimab.
All patients were able to avoid further treatment. Common grade 1-2 adverse events included rash or dermatitis (in 31% of patients), pruritus (25%), fatigue (25%) and nausea (19%). No grade > or equal 3 adverse events were observed.
Although preliminary, in terms of number of patients included and follow-up duration, these results demonstrate the potential for the 5-10% of patients with dMMR rectal cancers of having the possibility of a curative treatment for their disease.
Comment: even when longer follow-up is needed, these results are really a milestone in the treatment of these subset of rectal cancer patients (dMMR patients). A deeper understanding of the mechanisms underlying these impressive clinical responses could facilitate improvements in the outcomes of patients with tumors of non-dMMR phenotypes, that is the most prevalent rectal Cancer patient’s population.
• Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon Cancer
Daniel Gandia M.D. reviewing Tie J et al. N Engl J Med 2022; 386:2261-2272
Patients with colorectal cancer stage II (no lymph nodes in the pathological specimen) are a group that in general terms has excellent prognosis (80 % of patients are cured by surgery alone) and the discussion of to give adjuvant chemotherapy (metastases preventive chemotherapy), is only reserved for those patients with high-risk histopathological features.
In the last 2 decades, free tumoral circulating DNA begin to emerge as an important tool to add to histopathology. Its “name” is liquid biopsy, term related to its plasma circulation.
Tie et al, indicated that using circulating tumor DNA (ctDNA) analysis to guide treatment decisions reduces the number of patients deemed to require adjuvant chemotherapy without compromising recurrence-free survival (RFS).
In this clinical trial, 455 colorectal cancer patients, stage II were randomly assigned (2:1) to ctDNA-guided management and the other group to standard management. The primary end point was 2-years RFS.
At a median follow-up of 37 months, fewer patients received adjuvant chemotherapy in the ctDNA-guided group (16% versus 28% with standard management). In the evaluation of 2-year RFS, ctDNA-guided management was non inferior to standard management. Three-years RFS was 87 % among ctDNA-positive patients who received adjuvant chemotherapy and 92.5% among ctDNA-negative patients who did not received treatment.
Take-Home-Message: ctDNA-guided management in the treatment of stage II colorectal cancer patients, reduced the use of adjuvant chemotherapy without compromising the RFS. This can be translated to other early stages tumors with high-risk histopathological features, for the decision or not of post-surgery (adjuvant) chemotherapy.
• RAS at 40: Update from the RAS Initiative
Daniel Gandia reviewing Nissley DV and Mc Cormick F. Cancer Discovery. April 2022; 895-898.
The RAS protooncogenes family, are the oldest ones found in nature. When mutated they are transformed in Oncogenes, and when these mutated, they can activate the Cancer cascade. This last, is related to several processes such as cell proliferation, cell dedifferentiation, apoptosis (programmed cell death), and metastases.
There are 3 classes of RAS: KRAS, HRAS and NRAS.
The KRAS is the more frequently studied at present and found mutated in many tumors. It is an object of study to be tackled with several novel drugs.
Two solid tumors, present KRAS as the oncogene driver of the oncogenesis: Non-Small Cell Lung Cancer (NSCLC) and Colorectal cancer.
Recently, as KRAS as the other members in-family, worked with GTP, it has been discovered a pocket called G12C where an amino acid is substituted by other. This renders KRAS as passible of being blocked by drugs.
Sotorasib, is the first-in-class compound that tackles the druggable pocket part of KRAS.
It has been tested in NSCLC and Colorectal Cancer, and in the first one, impressive tumoral responses has been found. Those patients even were pretreated ones.
In the meantime, another drug with efficacy in colorectal Cancer is on the way of being launched.
Comment: 40 years have passed since KRAS, and the other members of the family were discovered. RAS code proteins that are ubiquitous at the cell membrane and cytosol and each one activates the other one, with the result that the final Cancer pathways are switched on. We are on the way of knowing more about the G-domains of KRAS, and when discovering new mutations, these can be blocked by new study drugs studied by computational methods. Even when the scenario is a challenging one, we have hi hopes of an enormous molecular cell developments with immediate Clinical Cancer translations.
定大國際法律事務所
定大會計師事務所